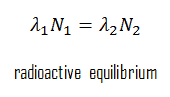In physics of nuclear decays, a radioactive equilibrium exists when a radioactive nuclide is decaying at the same rate at which it is being produced. The disintegrating nucleus is usually referred to as the parent nucleus and the nucleus remaining after the event as the daughter nucleus. The daughter nucleus can either be stable or radioactive. If it is radioactive, then it decays into a daughter nucleus and so on. Thus, each radioactive parent nucleus can initiate a series of decays, with each decay-product having its own characteristic decay constant.
Concentration of daughter nuclei in the radioactive equilibrium depends primarily on proportions of half-lives (or decay constants) of parent and daughter nuclei. Since the production rate and decay rate are equal, the number of atoms present remains constant over time. In any case, a radioactive equilibrium is not established immediately, but it only takes place after a transition period. This period is of the order of few half-lifes of the longest-lived nucleus in the decay chain. In case of radioactive decay chains, a radioactive equilibrium may be established between each member of the decay chain.
As was written, proportionality of half-lives is a key parameter, which determines type of radioactive equilibrium:
- Radioactive equilibrium is not established when a half-life of the parent nucleus is shorter than a half-life of the daughter nucleus. In this case the production rate and decay rate of certain member of decay chain cannot be equal.
- Secular radioactive equilibrium exists when the parent nucleus has an extremely long half-life. This type of equilibrium is particularly important in nature. Over the 4.5 billion years of the Earth’s history, especially uranium 238, uranium 235 and thorium 232 and members of their decay chains have reached radioactive equilibria between the parent nucleus and the various descendants.
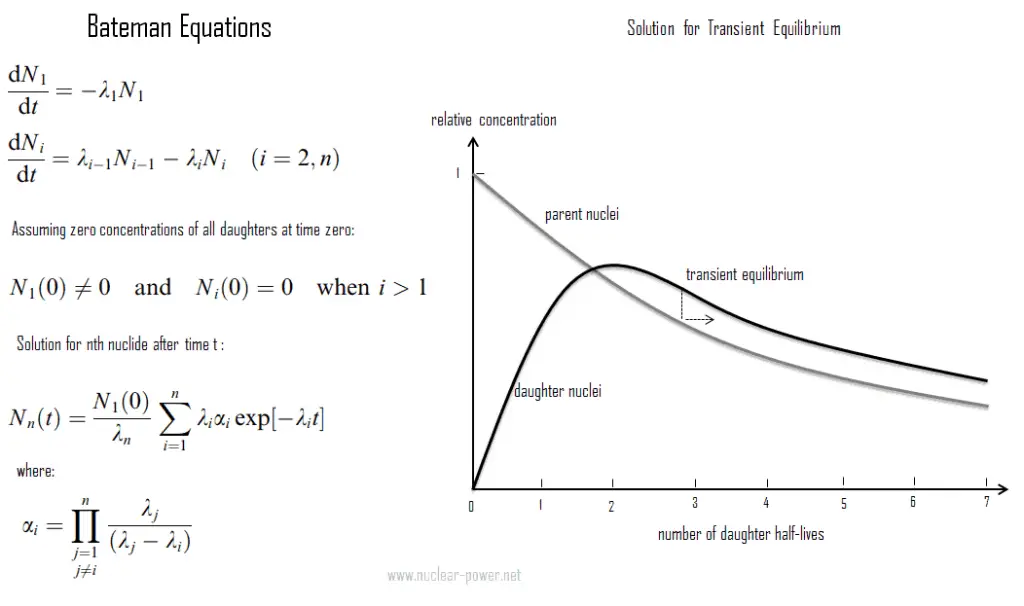
- Transient radioactive equilibrium exists when a half-life of the parent nucleus is longer than a half-life of the daughter nucleus. In this case, the parent nuclide and the daughter nuclide decay at essentially the same rate.
Transient Radioactive Equilibrium
The transient radioactive equilibrium exists when a half-life of the parent nucleus is longer than a half-life of the daughter nucleus, but the concentration of parent nuclei significantly decreases in time. In this case, the parent nuclide and the daughter nuclide may decay at essentially the same rate, but both concentrations of nuclides decreases as the concentration of parent nuclei decreases. Contrary to secular equilibrium, the half-life of the daughter nuclei is not negligible compared to parent’s half-life.
An example of this type of compound decay process is a Technetium-99m generator producing technetium-99m for nuclear medicine diagnostic procedures from molybdenum-99. Technetium-99m’s short half-life of 6 hours makes storage impossible and would make transport very expensive. Therefere, for medical purposes, molybdenum-99 is used to produce technetium-99m. These two isotopes are in the transient equilibrium. The decay constant for molybdenum-99 is considerably smaller than the decay constant for technetium-99m. Although the decay constant for molybdenum-99 is smaller, the actual rate of decay is initially larger than that of molybdenum-99 because of the great difference in their initial concentrations. As the concentration of the daughter increases, the rate of decay of the daughter will approach and eventually match the decay rate of the parent. When this occurs, they are said to be in the transient equilibrium. In case of Technetium-99m generator, transient equilibrium occurs after about four half-lives. Today, technetium-99m is the most utilized element in nuclear medicine and is employed in a wide variety of nuclear medicine imaging studies.
Also the transient equilibrium can occasionally be disrupted when one of the intermediary nuclei leaves the sample where its ancestors are confined.
Transient Radioactive Equilibrium with Source – Example
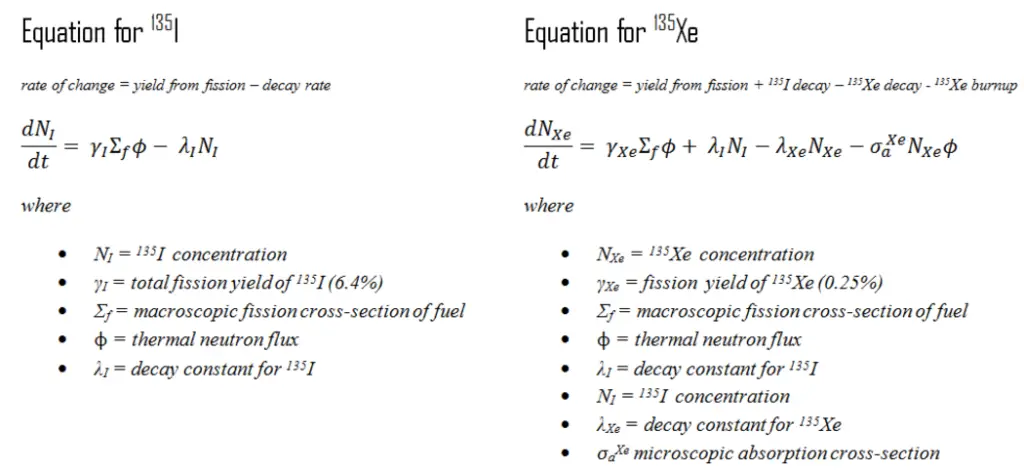
A special example of radioactive equilibrium are concentrations of iodine-135 and xenon-135 in a nuclear reactor, but in this case, the xenon burnup must be taken into account. Note that, in this special case, the half-life of the parent nucleus is shorter than the half-life of the daughter nucleus. The production and removal of xenon can be characterized by the following differential equations:
 When the rate of production of iodine equals the rate of removal of iodine, equilibrium exists. This equilibrium also known as “xenon 135 reservoir”, since all of this iodine must undergo a decay to xenon. In equilibrium, the iodine concentration remains constant and is designated NI(eq). The following equation for the iodine equilibrium concentration can be determined from the preceding equation by setting the dNI/dt =0. Since the equilibrium iodine concentration is proportional to the fission reaction rate, it is also proportional to reactor power level.
When the rate of production of iodine equals the rate of removal of iodine, equilibrium exists. This equilibrium also known as “xenon 135 reservoir”, since all of this iodine must undergo a decay to xenon. In equilibrium, the iodine concentration remains constant and is designated NI(eq). The following equation for the iodine equilibrium concentration can be determined from the preceding equation by setting the dNI/dt =0. Since the equilibrium iodine concentration is proportional to the fission reaction rate, it is also proportional to reactor power level.
When the rate of production of xenon 135 equals the rate of removal, equilibrium exists also for xenon. The xenon concentration remains constant and is designated NXe(eq). The following equation (1) for the xenon equilibrium concentration can be determined from the preceding equation by setting the dNXe/dt =0. For xenon 135 to be in equilibrium, iodine 135 must also be in equilibrium. Substituting the expression for equilibrium iodine 135 concentration into the equation for equilibrium xenon (1) results in the following (2).
 From this equation it can be seen that the equilibrium value for xenon 135 increases as power increases, because the numerator is proportional to the fission reaction rate. But the thermal flux is also in the denominator. Therefore, as the thermal flux exceeds some value, the xenon burnup begins to dominate, and at approximately 1015 neutrons.cm-2.s-1, the xenon-135 concentration approaches a limiting value. The equilibrium iodine 135 and xenon 135 concentrations as a function of neutron flux are illustrated in the following figure.
From this equation it can be seen that the equilibrium value for xenon 135 increases as power increases, because the numerator is proportional to the fission reaction rate. But the thermal flux is also in the denominator. Therefore, as the thermal flux exceeds some value, the xenon burnup begins to dominate, and at approximately 1015 neutrons.cm-2.s-1, the xenon-135 concentration approaches a limiting value. The equilibrium iodine 135 and xenon 135 concentrations as a function of neutron flux are illustrated in the following figure.
We hope, this article, Transient Equilibrium – Radioactive Equilibrium, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.