X-rays, also known as X-radiation, refers to electromagnetic radiation (no rest mass, no charge) of high energies. X-rays are high-energy photons with short wavelengths and thus very high frequency. The radiation frequency is key parameter of all photons, because it determines the energy of a photon. Photons are categorized according to the energies from low-energy radio waves and infrared radiation, through visible light, to high-energy X-rays and gamma rays.
Most X-rays have a wavelength ranging from 0.01 to 10 nanometers (3×1016 Hz to 3×1019 Hz), corresponding to energies in the range 100 eV to 100 keV. X-ray wavelengths are shorter than those of UV rays and typically longer than those of gamma rays.
Soft and Hard X-rays
X-rays are usually described by their maximum energy, which is determined by the voltage between the electrodes. X-rays with high photon energies (above 5–10 keV) are called hard X-rays, while those with lower energy (and longer wavelength) are called soft X-rays. Due to their penetrating ability, hard X-rays are widely used to image the inside of visually opaque objects. The most often seen applications are in medical radiography. Since the wavelengths of hard X-rays are similar to the size of atoms, they are also useful for determining crystal structures by X-ray crystallography. By contrast, soft X-rays are easily absorbed in air. The attenuation length of 600 eV X-rays in water is less than 1 micrometer.
X-ray – Production
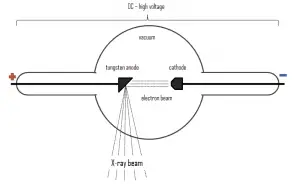 Since X-rays are high-energy photons, which have electromagnetic nature, they can be produced whenever charged particles (electrons or ions) of sufficient energy hit a material. It is similar to the photoelectric effect, where photons can be annihilated when they strike the metal plate, each one surrendering its kinetic energy to an electron.
Since X-rays are high-energy photons, which have electromagnetic nature, they can be produced whenever charged particles (electrons or ions) of sufficient energy hit a material. It is similar to the photoelectric effect, where photons can be annihilated when they strike the metal plate, each one surrendering its kinetic energy to an electron.
X-rays can be generated by an X-ray tube, a vacuum tube that uses a high voltage to accelerate the electrons released by a hot cathode to a high velocity. The cathode must be heated in order to emit electrons. Electrons, accelerated by potential differences of tens of thousands of volts, are aimed at a metal target (usually made of tungsten or another heavy metal) in a vacuum tube. The larger the voltage between the electrodes the higher energy will the electrons attain. Upon striking the target, the accelerated electrons are abruptly stopped and X-rays and heat are generated. Most of the energy is transformed into heat in the anode (which must be cooled). Just 1% of the kinetic energy of the electrons is converted into X-rays. X-rays are usually generated perpendicular to the path of the electron beam.
A specialized source of X-rays which is becoming widely used in research is particle accelerator, which generates radiation known as synchrotron radiation. When ultra-relativistic charged particles move through magnetic fields they are forced to move along a curved path. Since their direction of motion is continually changing, they are also accelerating and so emit bremsstrahlung, in this case it is referred to as synchrotron radiation.
X-rays can also be produced by fast protons or other positive ions. The proton-induced X-ray emission or particle-induced X-ray emission is widely used as an analytical procedure.
X-Ray Spectrum – Characteristic and Continuous
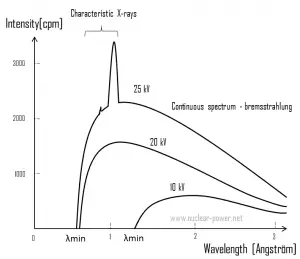 For X-rays generated by X-ray tube, the part of energy that is transformed into radiation varies from zero up to the maximum energy of the electron when it hits the anode. The maximum energy of the produced X-ray photon is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so an 100 kV tube cannot create X-rays with an energy greater than 100 keV. When the electrons hit the target, X-rays are created by two different atomic processes:
For X-rays generated by X-ray tube, the part of energy that is transformed into radiation varies from zero up to the maximum energy of the electron when it hits the anode. The maximum energy of the produced X-ray photon is limited by the energy of the incident electron, which is equal to the voltage on the tube times the electron charge, so an 100 kV tube cannot create X-rays with an energy greater than 100 keV. When the electrons hit the target, X-rays are created by two different atomic processes:
- Bremsstrahlung. The bremsstrahlung is electromagnetic radiation produced by the acceleration or deceleration of an electron when deflected by strong electromagnetic fields of target high-Z (proton number) nuclei. The name bremsstrahlung comes from the German. The literal translation is ‘braking radiation’. From classical theory, when a charged particle is accelerated or decelerated, it must radiate energy. The bremsstrahlung is one of possible interactions of light charged particles with matter (especially with high atomic numbers). These X-rays have a continuous spectrum. The intensity of the X-rays increases linearly with decreasing frequency, from zero at the energy of the incident electrons, the voltage on the X-ray tube. Changing the material from which the target in the tube is made has no effect on the spectrum of this continuous radiation. If we were to switch from a molybdenum target to a copper target, for example, all features of the x-ray spectrum would change except the cutoff wavelength.
- Characteristic X-ray emission. If the electron has enough energy it can knock an orbital electron out of the inner electron shell of a metal atom. Since the process leaves a vacancy in the electron energy level from which the electron came, the outer electrons of the atom cascade down to fill the lower atomic levels, and one or more characteristic X-rays are usually emitted. As a result, sharp intensity peaks appear in the spectrum at wavelengths that are a characteristic of the material from which the anode target is made. The frequencies of the characteristic X-rays can be predicted from the Bohr model.
We hope, this article, Soft X-Ray – Hard X-Ray, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.