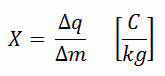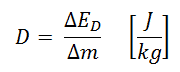External Dose Uptake
External exposure is radiation that comes from outside our body and interacts with us. In this case, we analyze predominantly exposure from gamma rays, since alpha and beta particles, in general, constitute no external exposure hazard because the particles generally do not pass through skin. The source of radiation can be, for example, a piece of equipment that produces the radiation like a container with a radioactive materials, or like an x-ray machine. In radiation protection there are three ways how to protect people from identified external radiation sources:
-
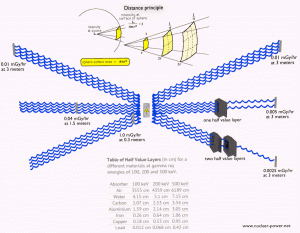
Principles of Radiation Protection – Time, Distance, Shielding Limiting Time. The amount of radiation exposure depends directly (linearly) on the time people spend near the source of radiation. The dose can be reduced by limiting exposure time.
- Distance. The amount of radiation exposure depends on the distance from the source of radiation. Similarly to a heat from a fire, if you are too close, the intensity of heat radiation is high and you can get burned. If you are at the right distance, you can withstand there without any problems and moreover it is comfortable. If you are too far from heat source, the insufficiency of heat can also hurt you. This analogy, in a certain sense, can be applied to radiation also from radiation sources.
- Shielding. Finally, if the source is too intensive and time or distance do not provide sufficient radiation protection, the shielding must be used. Radiation shielding usually consist of barriers of lead, concrete or water. There are many many materials, which can be used for radiation shielding, but there are many many situations in radiation protection. It highly depends on the type of radiation to be shielded, its energy and many other parametres. For example, even depleted uranium can be used as a good protection from gamma radiation, but on the other hand uranium is absolutely inappropriate shielding of neutron radiation.
As was written, it is crucial, whether we are exposed to radiation from external sources or from internal sources. This is similar as for another dangerous substances. Internal exposure is more dangerous than external exposure, since we are carrying the source of radiation inside our bodies and we cannot use any of radiation protection principles (time, distance, shielding).

Radiation Exposure
In general, radiation exposure is a measure of the ionization of air due to ionizing radiation from high-energy photons(i.e. X-rays and gamma rays). Radiation exposure is defined as the sum of electrical charges (∆q) on all the ions of one sign produced in air when all the electrons, liberated by photons in a volume of air whose mass is ∆m, are completely stopped in air.
Radiation exposure is given the symbol X. The SI unit of radiation exposure is the coulomb per kilogram (C/kg), but in practice, the roentgen is used. The roentgen, abbreviated R, is the unit of radiation exposure. In the original definition 1 R means the amount of X-rays or γ-radiation that is required to liberate positive and negative charges of one electrostatic unit of charge (esu) in 1 cm³ of dry air at standard temperature and pressure (STP).
Absorbed and Equivalent Dose
In radiation protection, the sievert is a derived unit of equivalent dose and effective dose. The sievert represents the equivalent biological effect of the deposit of a joule of gamma rays energy in a kilogram of human tissue. Absorbed dose is defined as the amount of energy deposited by ionizing radiation in a substance. Absorbed dose is given the symbol D. The absorbed dose is usually measured in a unit called the gray (Gy), which is derived from the SI system. The non-SI unit rad is sometimes also used, predominantly in the USA.
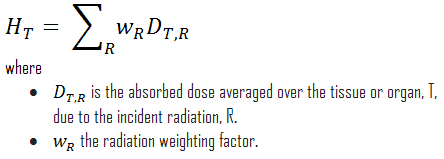
For radiation protection purposes, the absorbed dose is averaged over an organ or tissue, T, and this absorbed dose average is weighted for the radiation quality in terms of the radiation weighting factor, wR, for the type and energy of radiation incident on the body. The radiation weighting factor is a dimensionless factor used to determine the equivalent dose from the absorbed dose averaged over a tissue or organ and is based on the type of radiation absorbed. The resulting weighted dose was designated as the organ- or tissue equivalent dose:
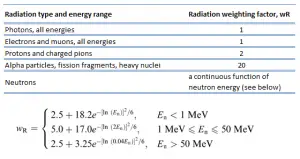
An equivalent dose of one Sievert represents that quantity of radiation dose that is equivalent, in terms of specified biological damage, to one gray of X-rays or gamma rays. A dose of one Svcaused by gamma radiation is equivalent to an energy deposition of one joule in a kilogram of a tissue. That means one sievert is equivalent to one gray of gamma rays deposited in certain tissue. On the other hand, similar biological damage (one sievert) can be caused only by 1/20 gray of alpha radiation (due to high wR of alpha radiation). Therefore, the sievert is not a physical dose unit. For example, an absorbed dose of 1 Gy by alpha particles will lead to an equivalent dose of 20 Sv. This may seem to be a paradox. It implies that the energy of the incident radiation field in joules has increased by a factor of 20, thereby violating the laws of Conservation of energy. However, this is not the case. Sievert is derived from the physical quantity absorbed dose, but also takes into account the biological effectiveness of the radiation, which is dependent on the radiation type and energy. The radiation weighting factor causes that the sievert cannot be a physical unit.
We hope, this article, External Exposure – External Contamination, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.