Atoms are the smallest constituents of ordinary matter, which can be divided without the release of electrically charged particles. The atoms consist of two parts. An atomic nucleus and an electron cloud. Radioactive atoms are atoms, which contain unstable nucleus, and which may undergo radioactive decay. The term radioactive atom is misleading term, since usually only nuclei may undergo decay and changes in electron configuration are only a result of changes in nucleus configuration. It must be noted, this is not a rule. In case of electron capture, also electron cloud plays a key role, since a parent nucleus must capture one of its orbital electrons.
Radionuclides
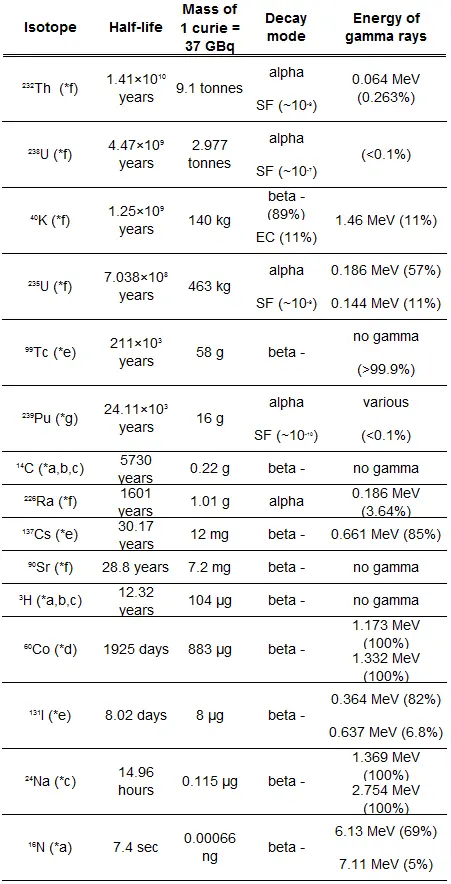
There are nuclides that are unstable and radioactive. These nuclides are known as radionuclides (radioactive nuclides) or radioisotopes (radioactive isotopes). These unstable isotopes decay through various radioactive decay pathways, most commonly alpha decay, beta decay, gamma decay or electron capture. Many other rare types of decay, such as spontaneous fission or neutron emission are known.
As was written, radioactive decay of radionuclides is a random process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay. In other words, a nucleus of a radionuclide has no “memory”. A nucleus does not “age” with the passage of time. Thus, the probability of its breaking down does not increase with time, but stays constant no matter how long the nucleus has existed.
Therefore, the rate of nuclear decay can be also measured in terms of half-lives. Each radionuclide has its own particular half-life that never changes, regardless of the quantity or form of the material (i.e., solid, liquid, gas, element or compound) or its past history. If a radioisotope has a half-life of 14 days, half of its atoms will have decayed within 14 days. In 14 more days, half of that remaining half will decay, and so on.
We hope, this article, Radioactive Atom, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.