Ionization Chambers
The ionization chamber, also known as the ion chamber, is electrical device that detects various types of ionizing radiation. The voltage of detector is adjusted so that the conditions correspond to the ionization region. The voltage is not high enough to produce gas amplification (secondary ionization).
Advantages of Ionization Chambers
- Current mode. Ionization chambers are preferred for high radiation dose rates because they have no “dead time”, a phenomenon which affects the accuracy of the Geiger-Mueller tube at high dose rates. This is due to the fact, there is no inherent amplification of signal in the operating medium and therefore these types of counters do not require much time to recover from large currents. In addition, because there is no amplification, they provide excellent energy resolution, which is limited primarily by electronic noise. Ionization chambers can be operated in current or pulse mode. In contrast, proportional counters or Geiger counters are almost always used in pulse mode. Detectors of ionizing radiation can be used both for activity measurements as well as for dose measurement. With knowledge about the energy needed to form an pair of ions – the dose can be obtained. The flat plate design is preferred because it has a well-defined active volume and ensures that ions will not collect on the insulators and cause a distortion of the electric field.
- Simplicity. Output current is independent of detector operating voltage. Observe the flat region of the curve in the ion chamber region. As a result, less regulated and thereby less expensive and more portable power supplies can be used with ion chamber instruments, and still offer a reasonably accurate response.
- Neutron Detection. In nuclear reactors, ionization chambers in current mode are often used to detect neutrons and belong to the Neutron Instrumentation System (NIS). For example, if the inner surface of the ionization chamber is coated with a thin coat of boron, the (n,alpha) reaction can take place. Most of (n,alpha) reactions of thermal neutrons are 10B(n,alpha)7Li reactions accompanied by 0.48 MeV gamma emission. Moreover, isotope boron-10 has high (n,alpha) reaction cross-section along the entire neutron energy spectrum. The alpha particle causes ionization within the chamber, and ejected electrons cause further secondary ionizations. Another method for detecting neutrons using an ionization chamber is to use the gas boron trifluoride (BF3) instead of air in the chamber. The incoming neutrons produce alpha particles when they react with the boron atoms in the detector gas. Either method may be used to detect neutrons in nuclear reactor.
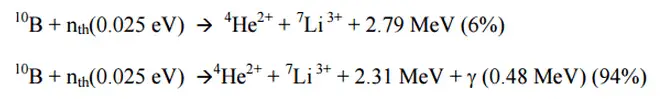
Disadvantages of Ionization Chambers
- No Charge Amplification. Detectors in the ionization region operate at a low electric field strength, selected such that no gas multiplication takes place. The charge collected (output signal) is independent of the applied voltage and for single minimum-ionizing particles tends to be quite small and usually require special low-noise amplifiers for attaining efficient operating performance. In air, the average energy needed to produce an ion is about 34 eV, therefore a 1 MeV radiation completely absorbed in the detector produces about 3 x 104 pair of ions. However it is a small signal, this signal can be considerably amplified using standard electronics. A current of 1 micro-ampere consists of about 1012 electrons per second.
- Low Density. Gamma rays deposit significantly lower amount of energy to the detector than other particles. The efficiency of the chamber can be further increased by the use of a high pressure gas.
- For alpha and beta particles to be detected by ionization chambers, they must be provided with a thin window. This “end-window” must be thin enough for the alpha and beta particles to penetrate. However, a window of almost any thickness will prevent an alpha particle from entering the chamber. The window is usually made of mica with a density of about 1.5 – 2.0 mg/cm2.
Semiconductor Detectors
A semiconductor detector is a radiation detector which is based on a semiconductor, such as silicon or germanium to measure the effect of incident charged particles or photons. Semiconductor detectors are widely used in radiation protection, assay of radioactive materials and physics research because they have some unique features, can be made inexpensively yet with good efficiency, and can measure both the intensity and the energy of incident radiation. These detectors are employed to measure the energy of the radiation and for identification of particles. Of the available semiconductor materials, silicon is mainly used for charged particle detectors (especially for tracking charged particles) and soft X-ray detectors while germanium is widely used for gamma ray spectroscopy.
Advantages of HPGe Detectors
- Higher atomic number. Germanium is preferred due to its atomic number being much higher than silicon and which increases the probability of gamma ray interaction.
- Germanium has lower average energy necessary to create an electron-hole pair, which is 3.6 eV for silicon and 2.9 eV for germanium.
- Very good energy resolution. The FWHM for germanium detectors is a function of energy. For a 1.3 MeV photon, the FWHM is 2.1 keV, which is very low.
- Large Crystals. While silicon-based detectors cannot be thicker than a few millimeters, germanium can have a depleted, sensitive thickness of centimeters, and therefore can be used as a total absorption detector for gamma rays up to few MeV.
Disadvantages of HPGe Detectors
- Cooling. The major drawback of HPGe detectors is that they must be cooled to liquid nitrogen temperatures. Because germanium has relatively low band gap, these detectors must be cooled in order to reduce the thermal generation of charge carriers to an acceptable level. Otherwise, leakage current induced noise destroys the energy resolution of the detector. Recall, the band gap (a distance between valence and conduction band) is very low for germanium (Egap= 0.67 eV). Cooling to liquid nitrogen temperature (-195.8°C; -320°F) reduces thermal excitations of valence electrons so that only a gamma ray interaction can give an electron the energy necessary to cross the band gap and reach the conduction band.
- Price. The disadvantage is that germanium detectors are much more expensive than ionization chambers or scintillation counters.
Advantages of Silicon Detectors
- Compared with gaseous ionization detectors, the density of a semiconductor detector is very high, and charged particles of high energy can give off their energy in a semiconductor of relatively small dimensions.
- Silicon has a high density of 2.329 g/cm3 and therefore the average energy loss per unit of length allows building thin detectors (e.g. 300 µm) that still produce measurable signals. For example, in case of minimum ionizing particle (MIP) the energy loss is 390 eV/µm. The silicon detectors are mechanically rigid and therefore no special supporting structures are needed.
- Silicon-based detectors are very good for tracking charged particles, they constitute a substantial part of detection system at the LHC in CERN.
- Silicon detectors can be used in strong magnetic fields.
Disadvantages of Silicon Detectors
- Price. The disadvantage is that silicon detectors are much more expensive than cloud chambers or wire chambers.
- Degradation. They also suffer degradation over time from radiation, however this can be greatly reduced thanks to the Lazarus effect.
- High FWHM. In gamma spectroscopy, germanium is preferred due to its atomic number being much higher than silicon and which increases the probability of gamma ray interaction. Moreover, germanium has lower average energy necessary to create an electron-hole pair, which is 3.6 eV for silicon and 2.9 eV for germanium. This also provides the latter a better resolution in energy.
We hope, this article, Ionization Chamber vs Semiconductor Detector, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.