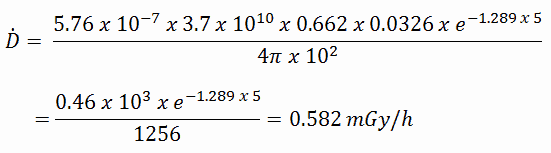Basic principles of radiation protection
In radiation protection there are three ways how to protect people from identified radiation sources:
- Limiting Time. The amount of radiation exposure depends directly (linearly) on the time people spend near the source of radiation. The dose can be reduced by limiting exposure time.
- Distance. The amount of radiation exposure depends on the distance from the source of radiation. Similarly to a heat from a fire, if you are too close, the intensity of heat radiation is high and you can get burned. If you are at the right distance, you can withstand there without any problems and moreover it is comfortable. If you are too far from heat source, the insufficiency of heat can also hurt you. This analogy, in a certain sense, can be applied to radiation also from radiation sources.
- Shielding. Finally, if the source is too intensive and time or distance do not provide sufficient radiation protection, the shielding must be used. Radiation shielding usually consist of barriers of lead, concrete or water. There are many many materials, which can be used for radiation shielding, but there are many many situations in radiation protection. It highly depends on the type of radiation to be shielded, its energy and many other parametres. For example, even depleted uranium can be used as a good protection from gamma radiation, but on the other hand uranium is absolutely inappropriate shielding of neutron radiation.
Characteristics of Gamma Rays / Radiation
Key features of gamma rays are summarized in following few points:
- Gamma rays are high-energy photons (about 10 000 times as much energy as the visible photons), the same photons as the photons forming the visible range of the electromagnetic spectrum – light.
- Photons (gamma rays and X-rays) can ionize atoms directly (despite they are electrically neutral) through the Photoelectric effect and the Compton effect, but secondary (indirect) ionization is much more significant.
- Gamma rays ionize matter primarily via indirect ionization.
- Although a large number of possible interactions are known, there are three key interaction mechanisms with matter.
- Gamma rays travel at the speed of light and they can travel thousands of meters in air before spending their energy.
- Since the gamma radiation is very penetrating matter, it must be shielded by very dense materials, such as lead or uranium.
- The distinction between X-rays and gamma rays is not so simple and has changed in recent decades. According to the currently valid definition, X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus.
- Gamma rays frequently accompany the emission of alpha and beta radiation.

Source: Wikimedia Commons
Shielding of Gamma Radiation
In short, effective shielding of gamma radiation is in most cases based on use of materials with two following material properties:
- high-density of material.
- high atomic number of material (high Z materials)
However, low-density materials and low Z materials can be compensated with increased thickness, which is as significant as density and atomic number in shielding applications.
A lead is widely used as a gamma shield. Major advantage of lead shield is in its compactness due to its higher density. On the other hand depleted uranium is much more effective due to its higher Z. Depleted uranium is used for shielding in portable gamma ray sources.
In nuclear power plants shielding of a reactor core can be provided by materials of reactor pressure vessel, reactor internals (neutron reflector). Also heavy concrete is usually used to shield both neutrons and gamma radiation.
Although water is neither high density nor high Z material, it is commonly used as gamma shields. Water provides a radiation shielding of fuel assemblies in a spent fuel pool during storage or during transports from and into the reactor core.
In general, the gamma radiation shielding is more complex and difficult than the alpha or beta radiation shielding. In order to understand comprehensively the way how a gamma ray loses its initial energy, how can be attenuated and how can be shielded we must have detailed knowledge of the its interaction mechanisms.
See also more theory: Interaction of Gamma Radiation with Matter
See also calculator: Gamma activity to dose rate (with/without shield)
See also XCOM – photon cross-section DB: XCOM: Photon Cross Sections Database
Gamma Rays Attenuetion
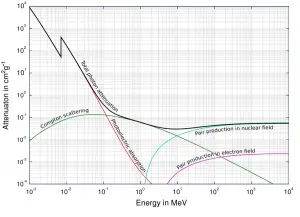
The total cross-section of interaction of a gamma rays with an atom is equal to the sum of all three mentioned partial cross-sections:σ = σf + σC + σp
- σf – Photoelectric effect
- σC – Compton scattering
- σp – Pair production
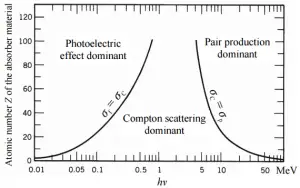
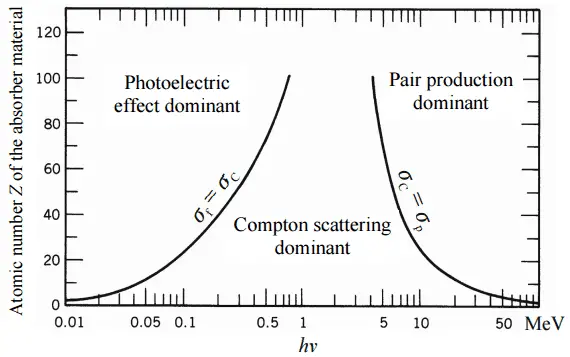
Depending on the gamma ray energy and the absorber material, one of the three partial cross-sections may become much larger than the other two. At small values of gamma ray energy the photoelectric effect dominates. Compton scattering dominates at intermediate energies. The compton scattering also increases with decreasing atomic number of matter, therefore the interval of domination is wider for light nuclei. Finally, electron-positron pair production dominates at high energies.
Based on the definition of interaction cross-section, the dependence of gamma rays intensity on thickness of absorber material can be derive. If monoenergetic gamma rays are collimated into a narrow beam and if the detector behind the material only detects the gamma rays that passed through that material without any kind of interaction with this material, then the dependence should be simple exponential attenuation of gamma rays. Each of these interactions removes the photon from the beam either by absorbtion or by scattering away from the detector direction. Therefore the interactions can be characterized by a fixed probability of occurance per unit path length in the absorber. The sum of these probabilities is called the linear attenuation coefficient:
μ = τ(photoelectric) + σ(Compton) + κ(pair)
Linear Attenuation Coefficient
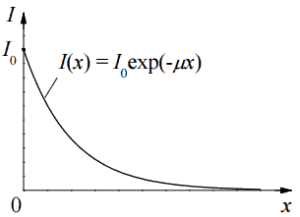
The attenuation of gamma radiation can be then described by the following equation.
I=I0.e-μx
, where I is intensity after attenuation, Io is incident intensity, μ is the linear attenuation coefficient (cm-1), and physical thickness of absorber (cm).
The materials listed in the table beside are air, water and a different elements from carbon (Z=6) through to lead (Z=82) and their linear attenuation coefficients are given for three gamma ray energies. There are two main features of the linear attenuation coefficient:
- The linear attenuation coefficient increases as the atomic number of the absorber increases.
- The linear attenuation coefficient for all materials decreases with the energy of the gamma rays.
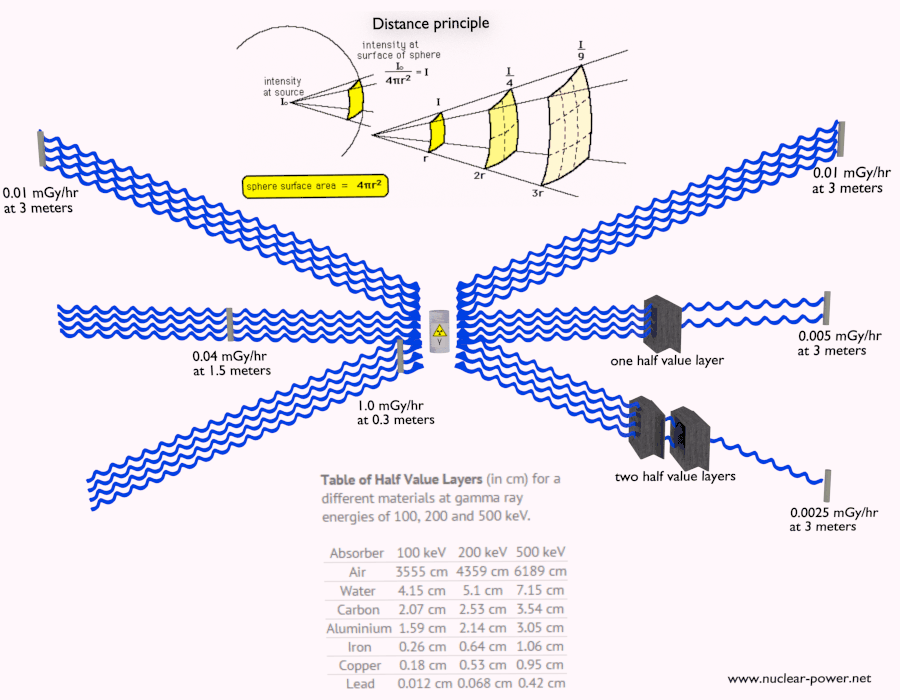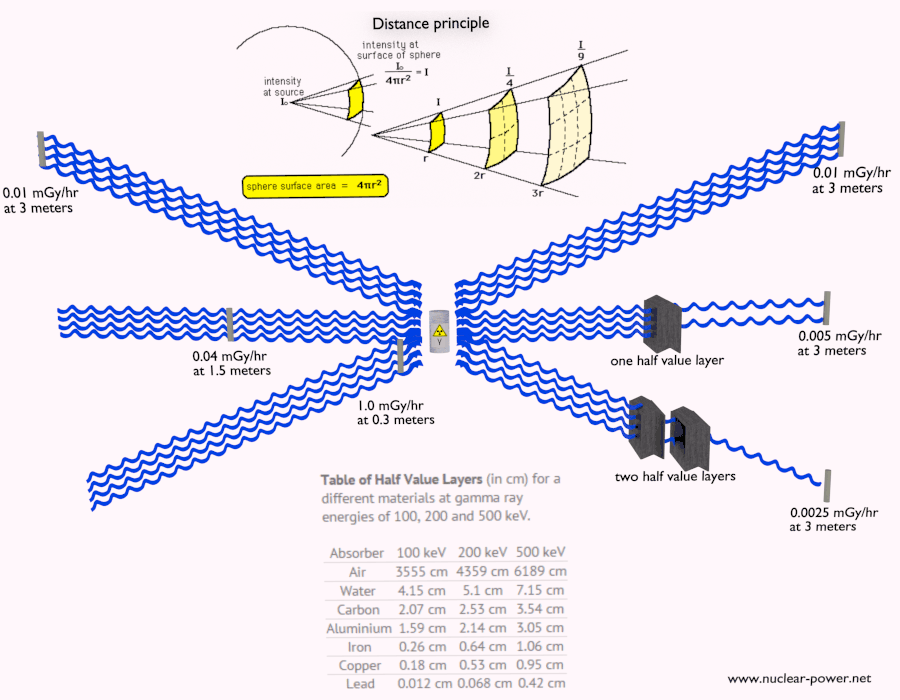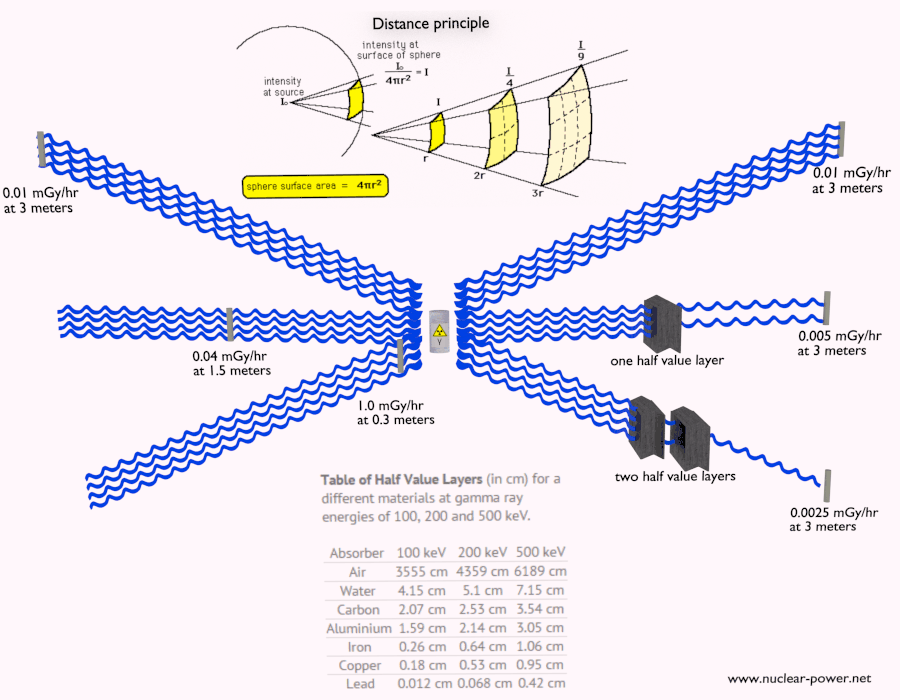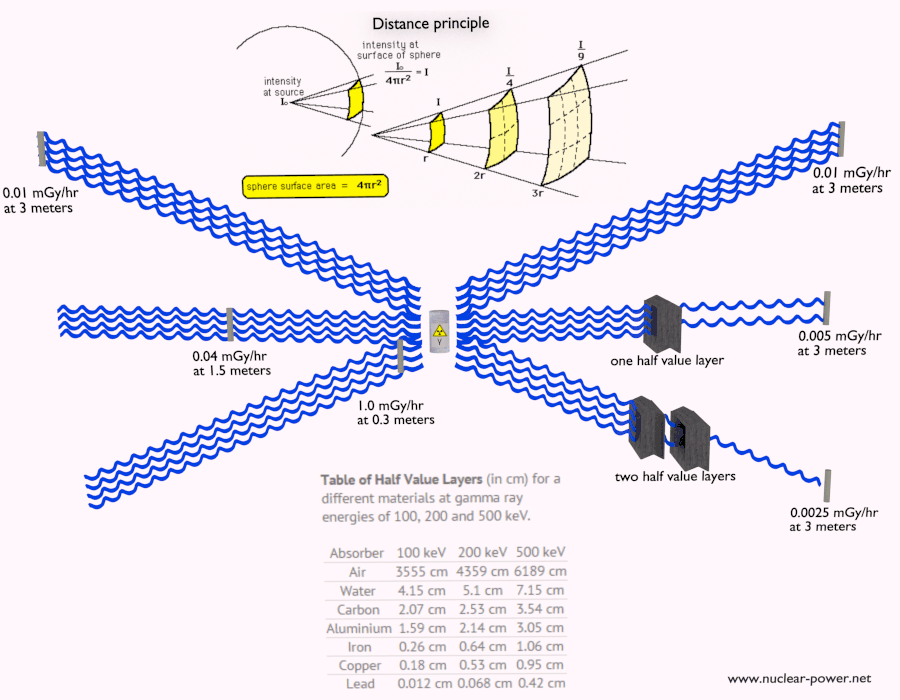
Half Value Layer
The half value layer expresses the thickness of absorbing material needed for reduction of the incident radiation intensity by a factor of two. There are two main features of the half value layer:
- The half value layer decreases as the atomic number of the absorber increases. For example 35 m of air is needed to reduce the intensity of a 100 keV gamma ray beam by a factor of two whereas just 0.12 mm of lead can do the same thing.
- The half value layer for all materials increases with the energy of the gamma rays. For example from 0.26 cm for iron at 100 keV to about 1.06 cm at 500 keV.
Example:
How much water schielding do you require, if you want to reduce the intensity of a 500 keV monoenergetic gamma ray beam (narrow beam) to 1% of its incident intensity? The half value layer for 500 keV gamma rays in water is 7.15 cm and the linear attenuation coefficient for 500 keV gamma rays in water is 0.097 cm-1.The question is quite simple and can be described by following equation:If the half value layer for water is 7.15 cm, the linear attenuation coefficient is:
Now we can use the exponential attenuation equation:
therefore
So the required thickness of water is about 47.5 cm. This is relatively large thickness and it is caused by small atomic numbers of hydrogen and oxygen. If we calculate the same problem for lead (Pb), we obtain the thickness x=2.8cm.
Linear Attenuation Coefficients
Table of Linear Attenuation Coefficients (in cm-1) for a different materials at gamma ray energies of 100, 200 and 500 keV.
| Absorber | 100 keV | 200 keV | 500 keV |
| Air | 0.000195/cm | 0.000159/cm | 0.000112/cm |
| Water | 0.167/cm | 0.136/cm | 0.097/cm |
| Carbon | 0.335/cm | 0.274/cm | 0.196/cm |
| Aluminium | 0.435/cm | 0.324/cm | 0.227/cm |
| Iron | 2.72/cm | 1.09/cm | 0.655/cm |
| Copper | 3.8/cm | 1.309/cm | 0.73/cm |
| Lead | 59.7/cm | 10.15/cm | 1.64/cm |
Half Value Layers
The half value layer expresses the thickness of absorbing material needed for reduction of the incident radiation intensity by a factor of two. With half value layer it is easy to perform simple calculations.
Source: www.nde-ed.org
Table of Half Value Layers (in cm) for a different materials at gamma ray energies of 100, 200 and 500 keV.
| Absorber | 100 keV | 200 keV | 500 keV |
| Air | 3555 cm | 4359 cm | 6189 cm |
| Water | 4.15 cm | 5.1 cm | 7.15 cm |
| Carbon | 2.07 cm | 2.53 cm | 3.54 cm |
| Aluminium | 1.59 cm | 2.14 cm | 3.05 cm |
| Iron | 0.26 cm | 0.64 cm | 1.06 cm |
| Copper | 0.18 cm | 0.53 cm | 0.95 cm |
| Lead | 0.012 cm | 0.068 cm | 0.42 cm |
Mass Attenuation Coefficient
When characterizing an absorbing material, we can use sometimes the mass attenuation coefficient. The mass attenuation coefficient is defined as the ratio of the linear attenuation coefficient and absorber density (μ/ρ). The attenuation of gamma radiation can be then described by the following equation:
I=I0.e-(μ/ρ).ρl
, where ρ is the material density, (μ/ρ) is the mass attenuation coefficient and ρ.l is the mass thickness. The measurement unit used for the mass attenuation coefficient cm2g-1.
For intermediate energies the Compton scattering dominates and different absorbers have approximately equal mass attenuation coefficients. This is due to the fact that cross section of Compton scattering is proportional to the Z (atomic number) and therefore the coefficient is proportional to the material density ρ. At small values of gamma ray energy or at high values of gamma ray energy, where the coefficient is proportional to higher powers of the atomic number Z (for photoelectric effect σf ~ Z5; for pair production σp ~ Z2), the attenuation coefficient μ is not a constant.
Validity of Exponential Law
The exponential law will always describe the attenuation of the primary radiation by matter. If secondary particles are producedor if the primary radiation changes its energy or direction, then the effective attenuation will be much less. The radiation will penetrate more deeply into matter than ispredicted by the exponential law alone. The processmust be taken into account whenevaluating the effect of radiation shielding.
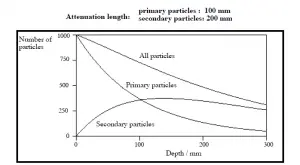
Buildup Factors for Gamma Rays Shielding
The buildup factor is a correction factor that considers the influence of the scattered radiation plus any secondary particles in the medium during shielding calculations. If we want to account for the buildup of secondary radiation, then we have to include the buildup factor. The buildup factor is then a multiplicative factor which accounts for the response to the uncollided photons so as to include the contribution of the scattered photons. Thus, the buildup factor can be obtained as a ratio of the total dose to the response for uncollided dose.
The extended formula for the dose rate calculation is:
The ANSI/ANS-6.4.3-1991 Gamma-Ray Attenuation Coefficients and Buildup Factors for Engineering Materials Standard, contains derived gamma-ray attenuation coefficients and buildup factors for selected engineering materials and elements for use in shielding calculations (ANSI/ANS-6.1.1, 1991).
We hope, this article, Shielding of Gamma Radiation, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.