We must note that radiation is all around us. In, around, and above the world we live in. It is a natural energy force that surrounds us. It is a part of our natural world that has been here since the birth of our planet. Whether the source of radiation is natural or man-made, whether it is a large dose of radiation or a small dose, there will be some biological effects. In general, ionizing radiation is harmful and potentially lethal to living beings but can have health benefits in medicine, for example, in radiation therapy for the treatment of cancer and thyrotoxicosis. This chapter briefly summarizes the short and long term consequences which may result from exposure to radiation. But first we need to define the differences in the types of radiation and the differences in the affected tissues.
High-LET and Low-LET Radiation
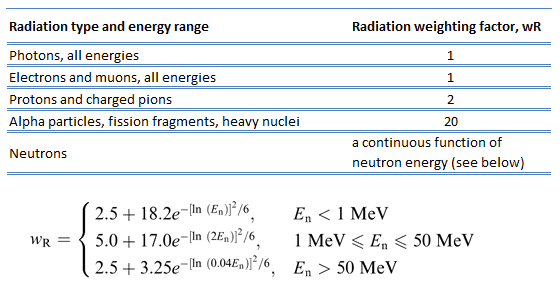
As was written, each type of radiation interacts with matter in a different way. For example charged particles with high energies can directly ionize atoms. Alpha particles are fairly massive and carry a double positive charge, so they tend to travel only a short distance and do not penetrate very far into tissue if at all. However alpha particles will deposit their energy over a smaller volume (possibly only a few cells if they enter a body) and cause more damage to those few cells.
Beta particles (electrons) are much smaller than alpha particles. They carry a single negative charge. They are more penetrating than alpha particles. They can travel several meters but deposit less energy at any one point along their paths than alpha particles. This means beta particles tend to damage more cells, but with lesser damage to each. On the other hand electrically neutral particles interacts only indirectly, but can also transfer some or all of their energies to the matter.
It would certainly simplify matters if biological effects of radiation were directly proportional to the absorbed dose. Unfortunately, biological effects depend also on the way in which the absorbed dose is distributed along the path of the radiation. Studies have shown that alpha and neutron radiation cause greater biological damage for a given energy deposition per kg of tissue than gamma radiation does. It was discovered, biological effects of any radiation increases with the linear energy transfer (LET). In short, the biological damage from high-LET radiation (alpha particles, protons or neutrons) is much greater than that from low-LET radiation (gamma rays). This is because the living tissue can more easily repair damage from radiation that is spread over a large area than that which is concentrated in a small area. Of course, at very high levels of exposure gamma rays can still cause a great deal of damage to tissues.
Because more biological damage is caused for the same physical dose (i.e., the same energy deposited per unit mass of tissue), one gray of alpha or neutron radiation is more harmful than one gray of gamma radiation. This fact that radiations of different types (and energies) give different biological effects for the same absorbed dose is described in terms of factors known as the relative biological effectiveness (RBE) and the radiation weighting factor (wR).
Cellular Damage – Radiation
All biological damage effects begin with the consequence of radiation interactions with the atoms forming the cells. All living things are composed of one or more cells. Every part of your body consists of cells or was built by them. Although we tend to think of biological effects in terms of the effect of radiation on living cells, in actuality, ionizing radiation, by definition, interacts only with atoms by a process called ionization. For ionizing radiation, the kinetic energy of particles (photons, electrons, etc.) of ionizing radiation is sufficient and the particle can ionize (to form ion by losing electrons) target atoms to form ions. Simply ionizing radiation can knock electrons from an atom.
There are two mechanisms by which radiation ultimately affects cells. These two mechanisms are commonly called:
- Direct effects. Direct effects are caused by radiation, when radiation interacts directly with the atoms of the DNA molecule, or some other cellular component critical to the survival of the cell. The probability of the radiation interacting with the DNA molecule is very small since these critical components make up such a small part of the cell.
- Indirect effects. Indirect effects are caused by interaction of radiation usually with water molecules. Each cell, just as is the case for the human body, is mostly water. Ionizing radiation may break the bonds that hold the water molecule together, producing radicals such as hydroxyl OH, superoxide anion O2– and others. These radicals can contribute to the destruction of the cell.
A large number of cells of any particular type is called a tissue. If this tissue forms a specialised functional unit, it is called an organ. The type and number of cells affected is also an important factor. Some cells and organs in the body are more sensitive to ionizing radiation than others.
Sensitivity of various types of cells to ionizing radiation is very high for tissues consisting of cells that divide rapidly like those found in bone marrow, stomach, intestines, male and female reproductive organs, and developing fetuses. This is because dividing cells require correct DNA information in order for the cell’s offspring to survive. A direct interaction of radiation with an active cell could result in the death or mutation of the cell, whereas a direct interaction with the DNA of a dormant cell would have less of an effect.
As a result, living cells can be classified according to their rate of reproduction, which also indicates their relative sensitivity to radiation. As a result, actively reproducing cells are more sensitive to ionizing radiation than cells that make up skin, kidney or liver tissue. The nerve and muscle cells are the slowest to regenerate and are the least sensitive cells.
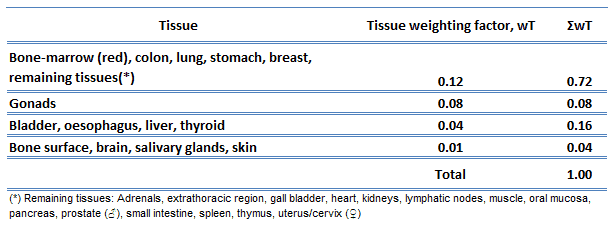 The sensitivity of the various organs of the human body correlate with the relative sensitivity of the cells from which they are composed. In practice, this sensitivity is represented by the tissue weighting factor, wT, which is the factor by which the equivalent dose in a tissue or organ T is weighted to represent the relative contribution of that tissue or organ to the total health detriment resulting from uniform irradiation of the body (ICRP 1991b).
The sensitivity of the various organs of the human body correlate with the relative sensitivity of the cells from which they are composed. In practice, this sensitivity is represented by the tissue weighting factor, wT, which is the factor by which the equivalent dose in a tissue or organ T is weighted to represent the relative contribution of that tissue or organ to the total health detriment resulting from uniform irradiation of the body (ICRP 1991b).
If a person is irradiated only partially, the dose will depend strongly on the tissue, which was irradiated. For example, a 10 mSv gamma dose to the whole body and a 50 mSv dose to the thyroid is the same, in terms of risk, as a whole-body dose of 10 + 0.04 x 50 = 12 mSv.
Acute Dose and Chronic Dose
Biological effects of radiation and their consequences depends strongly on the level of dose rate obtained. Dose rate is a measure of radiation dose intensity (or strength). Low-level doses are common for everyday life. In the following points there are a few examples of radiation exposure, which can be obtained from various sources.
- 05 µSv – Sleeping next to someone
- 09 µSv – Living within 30 miles of a nuclear power plant for a year
- 1 µSv – Eating one banana
- 3 µSv – Living within 50 miles of a coal power plant for a year
- 10 µSv – Average daily dose received from natural background
- 20 µSv – Chest X-ray
From biological consequences point of view, it is very important to distinguish between doses received over short and extended periods. Therefore, biological effects of radiation are typically divided into two categories.
- Acute Doses. An “acute dose” (short-term high-level dose) is one that occurs over a short and finite period of time, i.e., within a day.
- Chronic Doses. A “chronic dose” (long-term low-level dose) is a dose that continues for an extended period of time, i.e., weeks and months, so that it is better described by a dose rate.
High doses tend to kill cells, while low doses tend to damage or change them. High doses can cause visually dramatic radiation burns, and/or rapid fatality through acute radiation syndrome. Acute doses below 250 mGy are unlikely to have any observable effects. Acute doses of about 3 to 5 Gy have a 50% chance of killing a person some weeks after the exposure, if a person receives no medical treatment.
Low doses spread out over long periods of time don’t cause an immediate problem to any body organ. The effects of low doses of radiation occur at the level of the cell, and the results may not be observed for many years. Moreover, some studies demonstrate, most of human tissues exhibit a more pronounced tolerance to the effects of low-LET radiation in case of a prolonged exposure compared to a one-time exposure to a similar dose.
We hope, this article, Biological Effects of Radiation, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.