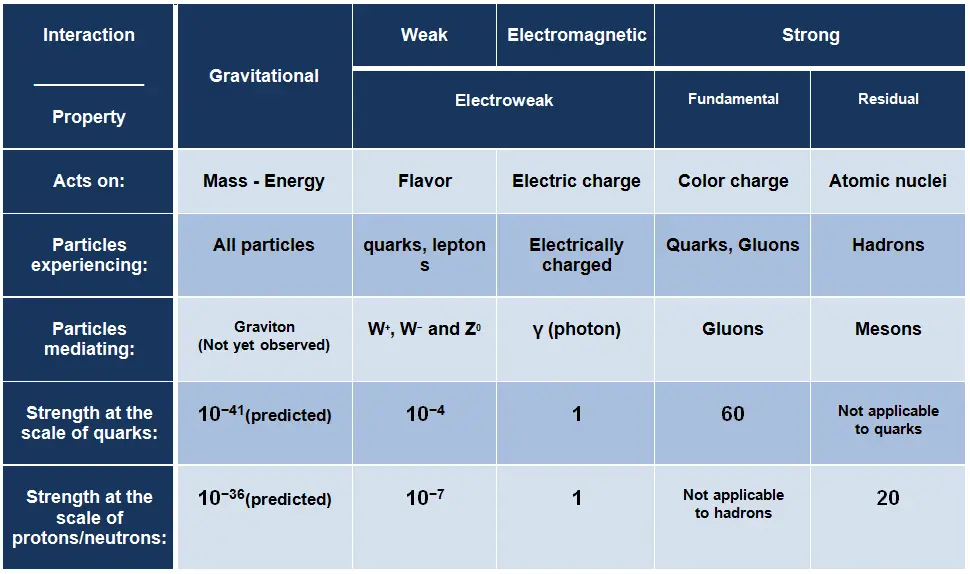
Strong Interaction – Strong Force
The strong interaction or strong force is one of the four fundamental forces and involves the exchange of the vector gauge bosons known as gluons. In general, the strong interaction is very complicated interaction, because it significantly varies with distance. The strong nuclear force holds most ordinary matter together because it confines quarks into hadron particles such as the proton and neutron. Moreover, the strong force is the force which can hold a nucleus together against the enormous forces of repulsion (electromagnetic force) of the protons is strong indeed. From this point of view, we have to distinguish between:
- Fundamental Strong Force. The fundamental strong force, or the strong force, is a very short range (less than about 0.8 fm, the radius of a nucleon) force, that acts directly between quarks. This force holds quarks together to form protons, neutrons, and other hadron particles. The strong interaction is mediated by the exchange of massless particles called gluons that act between quarks, antiquarks, and other gluons.
- Residual Strong Force. The residual strong force, also known as the nuclear force, is a very short range (about 1 to 3 fm) force, which acts to hold neutrons and protons together in nuclei. In nuclei, this force acts against the enormous repulsive electromagnetic force of the protons. The term residual is associated with the fact, it is the residuum of the fundamental strong interaction between the quarks that make up the protons and neutrons. The residual strong force acts indirectly through the virtual π and ρ mesons, which transmit the force between nucleons that holds the nucleus together.
Electromagnetic Interaction – Electromagnetic Force
The electromagnetic force is the force responsible for all electromagnetic processes. It acts between electrically charged particles. It is infinite-ranged force, much stronger than gravitational force, obeys the inverse square law, but neither electricity nor magnetism adds up in the way that gravitational force does. Since there are positive and negative charges (poles), these charges tend to cancel each other out. Electromagnetism includes the electrostatic force acting between charged particles at rest, and the combined effect of electric and magnetic forces acting between charged particles moving relative to each other.
The photon, the quantum of electromagnetic radiation, is an elementary particle, which is the force carrier of the electromagnetic force. Photons are gauge bosons having no electric charge or rest mass and one unit of spin. Common to all photons is the speed of light, the universal constant of physics. In empty space, the photon moves at c (the speed of light – 299 792 458 metres per second).
Forces between static electrically charged particles are governed by the Coulomb’s law. Coulomb’s Law can be used to calculate the force between charged particles (e.g. two protons). The electrostatic force is directly proportional to the electrical charges of the two particles and inversely proportional to the square of the distance between the particles. Coulomb’s Law is stated as the following equation.
Both, the Coulomb’s law and the magnetic force, are summarized in the Lorentz force law. Fundamentally, both magnetic and electric forces are manifestations of an exchange force involving the exchange of photons.
The electromagnetic force plays a major role in determining the internal properties of most objects encountered in daily life. The chemical properties of atoms and molecules are determined by the number of protons, in fact, by number and arrangement of electrons.
We hope, this article, Strong Force vs Electromagnetic Force, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.