Radiometric dating (or radioactive dating) is any technique used to date organic and also inorganic materials from a process involving radioactive decay. The method compares the abundance of a naturally occurring radioactive isotope within the material to the abundance of its decay products, which form at a known constant rate of decay.
All these methods are based on the fact the rate at which radioactive nuclei disintegrate is unaffected by their environment, it can be used to estimate the age of any material sample or object which contains a radioactive isotope. Calculations of the decay of radioactive nuclei are relatively straightforward, owing to the fact that there is only one fundamental law governing all decay process.
The radioactive decay law states that the probability per unit time that a nucleus will decay is a constant, independent of time. This constant is called the decay constant and is denoted by λ, “lambda”. This constant probability may vary greatly between different types of nuclei, leading to the many different observed decay rates. The radioactive decay of certain number of atoms (mass) is exponential in time.
Radioactive decay law: N = N0.e-λt
Among the best-known techniques are:
- carbon-14 dating,
- potassium–argon dating,
- uranium–lead dating.
Radiometric dating methods are used in geochronology to establish the geologic time scale and can be also used to date archaeological materials, including ancient artifacts.
Carbon-14 Dating – Radiocarbon Dating
Carbon-14 dating, known also as radiocarbon dating, is a method for determining the age of an object containing organic material by using the properties of radionuclide carbon-14. Radioactive carbon-14 has a half-life of 5730 years and undergoes β− decay, where the neutron is converted into a proton, an electron, and an electron antineutrino:
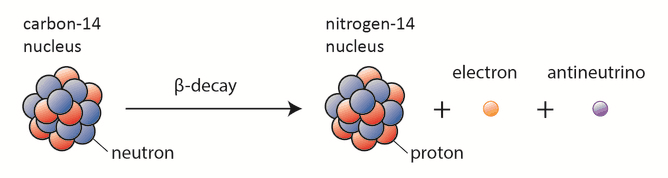
In spite of this short half-life compared to the age of the earth, carbon-14 is a naturally occurring isotope. Its presence can be explained by the following simple observation. Our atmosphere contains many gases, including nitrogen-14. Besides, the atmosphere is constantly bombarded with high energy cosmic rays, consisting of protons, heavier nuclei, or gamma rays. These cosmic rays interact with nuclei in the atmosphere, and produce also high-energy neutrons. These neutrons produced in these collisions can be absorbed by nitrogen-14 to produce an isotope of carbon-14:
Carbon-14 can also be produced in the atmosphere by other neutron reactions, including in particular 13C(n,γ)14C and 17O(n,α)14C. As a result, carbon-14 is continuously formed in the upper atmosphere by the interaction of cosmic rays with atmospheric nitrogen. On average just one out of every 1.3 x 1012 carbon atoms in the atmosphere is a radioactive carbon-14 atom.
The resulting carbon-14 combines with atmospheric oxygen to form radioactive carbon dioxide, which is incorporated into plants by photosynthesis. Consequently, all biological systems as plants, animals and humans contain a certain level of radioactive carbon-14. As long as the biological system is alive the level is constant due to constant intake of all isotopes of carbon. When the biological system dies, it stops exchanging carbon with its environment, and from that point onwards the amount of carbon-14 it contains begins to decrease as the carbon-14 undergoes radioactive decay. On the other hand, the amount of stable carbon-12 remains unchanged. As a result, the relative concentration of these two isotopes in any organism changes after its death. The method enables datings to be made up to about 20,000 years ago with an accuracy of about ±100 years.
The technique of carbon dating was suggested initially by Willard Libby and his colleagues in 1949. In 1960, Willard Libby was awarded the Nobel Prize in chemistry for this work.
Age of the Earth – Uranium-lead Dating
The age of the Earth is about 4.54 billion years. This dating is based on evidence from radiometric age-dating of meteorite material and is consistent with the radiometric ages of the oldest-known terrestrial and lunar samples.
One of the oldest radiometric dating methods is uranium-lead dating. The age of the earth’s crust can be estimated from the ratio between the amounts of uranium-238 and lead-206 found in geological specimens. The long half-life of the isotope uranium-238 (4.51×109 years) makes it well-suited for use in estimating the age of the earliest igneous rocks and for other types of radiometric dating, including uranium–thorium dating and uranium–uranium dating.
Uranium-lead dating is based on the measurement of the first and the last member of the uranium series, which is one of three classical radioactive series beginning with naturally occurring uranium-238. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of uranium series, the stable nucleus is lead-206. The assumption made is that all the lead-206 nuclei found in the specimen today were originally uranium-238 nuclei. That means at the crust’s formation the specimen contained no lead-206 nuclei. If no other lead isotopes are found in the specimen, this is a reasonable assumption. Under this condition, the age of the sample can be calculated by assuming exponential decay of uranium-238. That is:
Uranium-lead dating method is usually performed on the mineral zircon. Zircons from Jack Hills in Western Australia, have yielded U-Pb ages up to 4.404 billion years, interpreted to be the age of crystallization, making them the oldest minerals so far dated on Earth.
We hope, this article, Radiometric Dating – Radioactive Dating, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.
