Radioactive series (known also as a radioactive cascades) are three naturally occurring radioactive decay chains and one artificial radioactive decay chain of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. Most radioisotopes do not decay directly to a stable state and all isotopes within the series decay in the same way. In physics of nuclear decays, the disintegrating nucleus is usually referred to as the parent nucleus and the nucleus remaining after the event as the daughter nucleus. Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Within each series, therefore, the mass number of the members may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the thorium series is known as the 4n series, the neptunium series as the 4n + 1 series, the uranium series as the 4n + 2 series and the actinium series as the 4n + 3 series.
Three of the sets are called natural or classical series. The fourth set, the neptunium series, is headed by neptunium-237. Its members are produced artificially by nuclear reactions and do not occur naturally.
- the thorium series (4n series),
- the uranium series (4n+2 series),
- the actinium series (4n+3 series),
- the neptunium series (4n+1 series).
The classical series are headed by primordial unstable nuclei. Primordial nuclides are nuclides found on the Earth that have existed in their current form since before Earth was formed. The previous four series consist of the radioisotopes, that are the descendants of four heavy nuclei with long and very long half-lives:
- the thorium series with thorium-232 (with a half-life of 14.0 billion years),
- the uranium series with uranium-238 (which lives for 4.47 billion years),
- the actinium series with uranium-235 (with a half-life of 0.7 billion years).
- the neptunium series with neptunium-237 (with a half-life of 2 million years).
The half-lives of all the daughter nuclei are all extremely variable, and it is difficult to represent a range of timescales going from individual seconds to billions of years. Since daughter radioisotopes have different half-lives then secular equilibrium is reached after some time. In the long decay chain for a naturally radioactive element, such as uranium-238, where all of the elements in the chain are in secular equilibrium, each of the descendants has built up to an equilibrium amount and all decay at the rate set by the original parent. If and when equilibrium is achieved, each successive daughter isotope is present in direct proportion to its half-life. Since its activity is inversely proportional to its half-life, each nuclide in the decay chain finally contributes as many individual transformations as the head of the chain.
As can be seen from figures, branching occurs in all four of the radioactive series. That means the decay of a given species may occur in more than one way. For example, in the thorium series, bismuth-212 decays partially by negative beta emission to polonium-212 and partially by alpha emission to thallium-206.
Radioactive cascade significantly influences radioactivity (disintegrations per second) of natural samples and natural materials. All the descendants are present, at least transiently, in any natural sample, whether metal, compound, or mineral. For example, pure uranium-238 is weakly radioactive (proportional to its long half-life), but a uranium ore is about 13 times more radioactive than the pure uranium-238 metal because of its daughter isotopes (e.g. radon, radium etc.) it contains. Not only are unstable radium isotopes significant radioactivity emitters, but as the next stage in the decay chain they also generate radon, a heavy, inert, naturally occurring radioactive gas. Moreover the decay heat of uranium and its decay products (e.g. radon, radium etc.) contributes to heating of Earth’s core. Together with thorium and potassium-40 in the Earth’s mantle is thought that these elements are the main source of heat that keeps the Earth’s core liquid.
See also: Radioactive Equilibrium
Types of Decay
Within each radioactive series, there are two main modes of radioactive decay:
- Alpha decay. Alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleus. Because of its very large mass (more than 7000 times the mass of the beta particle) and its charge, it heavy ionizes material and has a very short range.
- Beta decay. Beta decay or β decay represents the disintegration of a parent nucleus to a daughter through the emission of the beta particle. Beta particles are high-energy, high-speed electrons or positrons emitted by certain types of radioactive nuclei such as potassium-40. The beta particles have greater range of penetration than alpha particles, but still much less than gamma rays.The beta particles emitted are a form of ionizing radiation also known as beta rays. The production of beta particles is termed beta decay.
Thorium Series
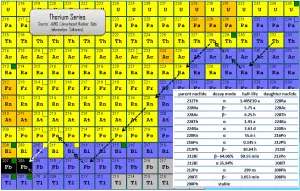 The thorium series is one of three classical radioactive series beginning with naturally occurring thorium-232. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of thorium series, the stable nucleus is lead-208.
The thorium series is one of three classical radioactive series beginning with naturally occurring thorium-232. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of thorium series, the stable nucleus is lead-208.
Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Within each series, therefore, the mass number of the members may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the thorium series is known as the 4n series.
The total energy released from thorium-232 to lead-208, including the energy lost to neutrinos, is 42.6 MeV.
Neptunium Series
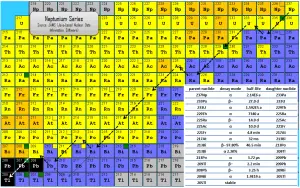 The neptunium series is a radioactive series beginning with neptunium-237. Its members are produced artificially by nuclear reactions and do not occur naturally, because the half-life of the longest lived isotope in the series is short compared to the age of the earth. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of neptunium series, the stable nucleus is bismuth-209 (with half-life of 1.9E19 years) and thallium-205.
The neptunium series is a radioactive series beginning with neptunium-237. Its members are produced artificially by nuclear reactions and do not occur naturally, because the half-life of the longest lived isotope in the series is short compared to the age of the earth. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of neptunium series, the stable nucleus is bismuth-209 (with half-life of 1.9E19 years) and thallium-205.
Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Within each series, therefore, the mass number of the members may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the neptunium series is known as the 4n+1 series.
The total energy released from neptunium-237 to thallium-205, including the energy lost to neutrinos, is 50.0 MeV.
In some kind of smoke detectors, you can meet radionuclides from this series. Ionization smoke detectors usually use a radioisotope, typically americium-241, to ionize air and to detect smoke. In this case americium-241 decays to neptunium-237 and is, in fact, a member of neptunium series.
Uranium Series
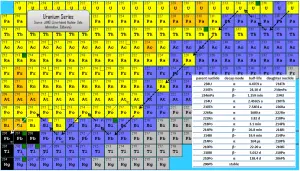 The uranium series, known also as radium series, is one of three classical radioactive series beginning with naturally occurring uranium-238. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of uranium series, the stable nucleus is lead-206.
The uranium series, known also as radium series, is one of three classical radioactive series beginning with naturally occurring uranium-238. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of uranium series, the stable nucleus is lead-206.
Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Within each series, therefore, the mass number of the members may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the uranium series is known as the 4n+2 series.
The total energy released from uranium-238 to lead-206, including the energy lost to neutrinos, is 51.7 MeV.
Uranium Series and Uranium-234
Isotope of uranium-234 is a member of this series. This isotope has the half-life of only 2.46×105 years and therefore it do not belong to primordial nuclides (unlike 235U and 238U). On the other hand, this isotope is still present in the Earth’s crust, but this is due to the fact 234U is an indirect decay product of 238U. 238U decays via alpha decay into 234U. 234U decays via alpha decay into 230Th, except very small fraction (on the order of ppm) of nuclei which decays by spontaneous fission.
In a natural sample of uranium, these nuclei are present in the unalterable proportions of the radioactive equilibrium of the 238U filiation at a ratio of one atom of 234U for about 18 500 nuclei of 238U. As a result of this equilibrium these two isotopes (238U and 234U) contribute equally to the radioactivity of natural uranium.
Actinium Series
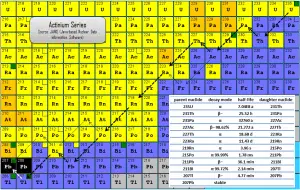 The actinium series is one of three classical radioactive series beginning with naturally occurring uranium-235. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of actinium series, the stable nucleus is lead-207.
The actinium series is one of three classical radioactive series beginning with naturally occurring uranium-235. This radioactive decay chain consists of unstable heavy atomic nuclei that decay through a sequence of alpha and beta decays until a stable nucleus is achieved. In case of actinium series, the stable nucleus is lead-207.
Since alpha decay represents the disintegration of a parent nucleus to a daughter through the emission of the nucleus of a helium atom (which contains four nucleons), there are only four decay series. Within each series, therefore, the mass number of the members may be expressed as four times an appropriate integer (n) plus the constant for that series. As a result, the actinium series is known as the 4n+3 series.
The total energy released from uranium-235 to lead-207, including the energy lost to neutrinos, is 46.4 MeV.
We hope, this article, Radioactive Series – Radioactive Cascade, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.