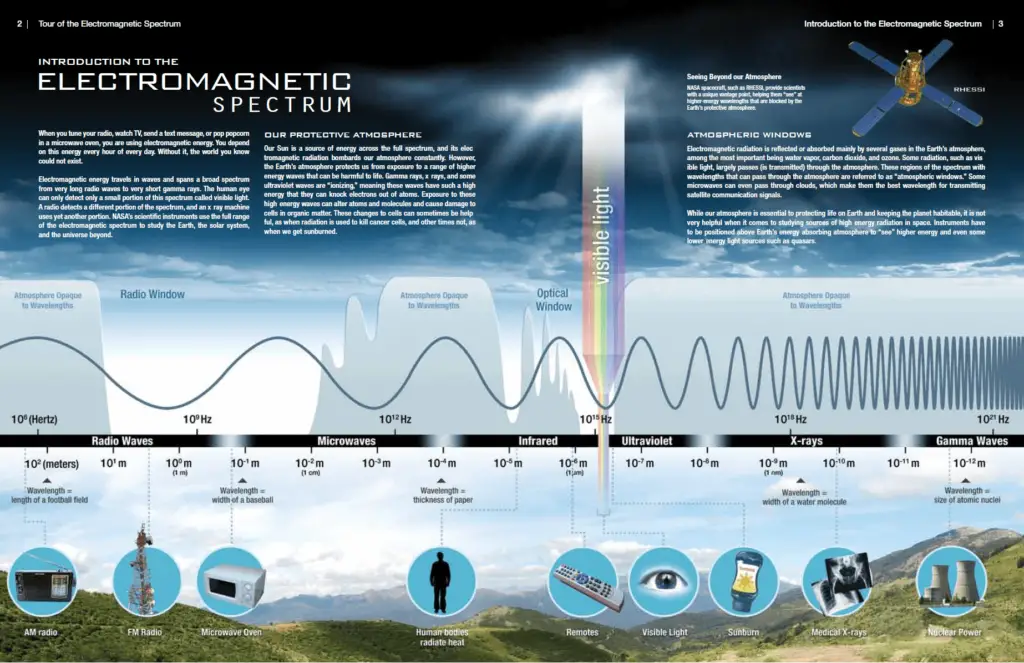
The distinction between X-rays and gamma rays is not so simple and has changed in recent decades. Both are high-energy photons (electromagnetic radiation) with very short wavelengths and thus very high frequency. Yes, X-rays are being said to have lower energies, but this is not the rule and the main difference. According to the currently valid definition, X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus.
Characteristics of X-rays
Key features of X-rays are summarized in following few points:
- X-rays are high-energy photons (about 100 – 1 000 times as much energy as the visible photons), the same photons as the photons forming the visible range of the electromagnetic spectrum – light.
- X-rays are usually described by their maximum energy, which is determined by the voltage between the electrodes. It may range from about 20 kV up to 300 kV. Radiation with low voltage is called “soft” – and radiation with high voltage is called “hard”.
- Photons have no mass and no electrical charge, therefore they cannot directly ionize matter, neither X-rays.
- X-rays ionize matter via indirect ionization.
- Although a large number of possible interactions are known, there are three key interaction mechanisms with matter.
- X-rays travel at the speed of light and they can travel hundreds of meters in air before spending their energy.
- Since the hard X-rays are very penetrating matter, it must be shielded by very dense materials, such as lead or uranium.
- The distinction between X-rays and gamma rays is not so simple and has changed in recent decades. According to the currently valid definition, X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus.
- For X-rays generated by X-ray tube, there are two different types of X-rays spectra:
- Characteristic X-rays frequently accompany some types of nuclear decays, such as internal conversion and electron capture.
Characteristics of Gamma Rays
Key features of gamma rays are summarized in following few points:
- Gamma rays are high-energy photons (about 10 000 times as much energy as the visible photons), the same photons as the photons forming the visible range of the electromagnetic spectrum – light.
- Photons have no mass and no electrical charge, therefore they cannot directly ionize matter, neither gamma rays.
- Despite this fact, gamma rays ionize matter via indirect ionization.
- Although a large number of possible interactions are known, there are three key interaction mechanisms with matter.
- Photoelectric effect
- Compton scattering
- Pair production
- Gamma rays travel at the speed of light and they can travel thousands of meters in air before spending their energy.
- Since the gamma radiation is very penetrating matter, it must be shielded by very dense materials, such as lead or uranium.
- The distinction between X-rays and gamma rays is not so simple and has changed in recent decades. According to the currently valid definition, X-rays are emitted by electrons outside the nucleus, while gamma rays are emitted by the nucleus.
- Gamma rays frequently accompany the emission of alpha and beta radiation.
We hope, this article, Gamma Ray – X-Ray – Difference – Distinction, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.