Carbon-14
The only cosmogenic radionuclide to make a significant contribution to internal exposure of human is carbon-14. Radioactive carbon-14 has a half-life of 5730 years and undergoes β− decay, where the neutron is converted into a proton, an electron, and an electron antineutrino:
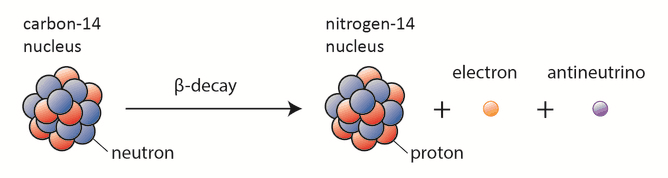
In spite of this short half-life compared to the age of the earth, carbon-14 is a naturally occurring isotope. Its presence can be explained by the following simple observation. Our atmosphere contains many gases, including nitrogen-14. Besides, the atmosphere is constantly bombarded with high energy cosmic rays, consisting of protons, heavier nuclei, or gamma rays. These cosmic rays interact with nuclei in the atmosphere, and produce also high-energy neutrons. These neutrons produced in these collisions can be absorbed by nitrogen-14 to produce an isotope of carbon-14:
Carbon-14 can also be produced in the atmosphere by other neutron reactions, including in particular 13C(n,γ)14C and 17O(n,α)14C. As a result, carbon-14 is continuously formed in the upper atmosphere by the interaction of cosmic rays with atmospheric nitrogen. On average just one out of every 1.3 x 1012 carbon atoms in the atmosphere is a radioactive carbon-14 atom. As a result, all living biological substances contain the same amount of C-14 per gram of carbon, that is 0.3 Bq of carbon-14 activity per gram of carbon. Carbon-14 is present in the human body (13kg of carbon in 70kg human) at a level of about 3700 Bq (0.1 μCi) with a biological half-life of 40 days. Note that, biological half-life is the time taken for the amount of a particular element in the body to decrease to half of its initial value due to elimination by biological processes alone. However, a carbon atom is in the genetic information of about half the cells, while potassium is not a component of DNA. The decay of a carbon-14 atom inside DNA in one person happens about 50 times per second, changing a carbon atom to one of nitrogen.
The annual dose from carbon-14 is estimated to be about 12 μSv/year.
As long as the biological system is alive the level is constant due to constant intake of all isotopes of carbon. When the biological system dies, it stops exchanging carbon with its environment, and from that point onwards the amount of carbon-14 it contains begins to decrease as the carbon-14 undergoes radioactive decay.
See also: Carbon-14 Dating
We hope, this article, Carbon-14 – Production – Properties – Decay, helps you. If so, give us a like in the sidebar. Main purpose of this website is to help the public to learn some interesting and important information about radiation and dosimeters.